As appeared on MDAtl.com.
Lung cancer continues as the leading cause of cancer-related deaths in the United States in men and the second leading cause in women.1 The role for accurate, timely and sensitive diagnostic procedures continues to evolve. With this, bronchoscopy became a proven safe and accurate method for diagnosis and staging of lung cancer.2
With the implementation of lung cancer screening in 2010, the number of pulmonary nodules and potential new lung cancer diagnoses has grown exponentially.3 Now, smaller peripheral nodules are being discovered, increasing the diagnostic dilemma for methods of safe, accurate biopsy and staging.
Serious complications with traditional bronchoscopy-guided biopsies have remained low (0.637%).4 However, for peripherally located lesions < 2.5 cm, the yield by traditional bronchoscopy was approximately 40%.4
Transthoracic needle biopsies have a significantly better accuracy, with a false negativity rate of 20-25%, but some literature describes complication rates ranging from 5% to 50%.5-7 Improving diagnostic yields for smaller peripheral nodules while maintaining low complication rates in a single diagnostic and staging procedure is a focus of many new technological advances in interventional pulmonology, including robotic bronchoscopy.
The first use of robotic surgeries began in 2000 with the da Vinci Surgical System, with a numerous applications ranging from general surgeries to thoracic.8 Electromagnetic Navigation Bronchoscopy (EMB) was one of the first iterations of a guided system to aid in the localization of smaller, peripheral lung lesions for bronchoscopy-guided biopsies with the goal to increase accuracy of biopsy while maintaining a low complication rate. Combined with radial EBUS, yield accuracy of peripheral nodules increased to 66% with lesions down to 1-2 cm.9
These combined bronchoscopy-based modalities and existing surgical robotic platforms created the groundwork for developing robotic bronchoscopy. This emerging technology focuses on maintaining direct airway visualization in smaller peripheral lesions at fourth generation airway and further. This provides an increased accuracy of diagnosis without losing the safety aspects of previous EMB biopsies.10
Two robotic bronchoscopy platforms were recently developed. The Monarch™ (MA; Auris Health, Redwood City, CA) and the Ion™ Endoluminal Platform (IEP; Intuitive, Sunnyvale, CA) received FDA approval for use in 2018 and 2019 respectively. Again, a key feature to both of these systems is allowing direct visualization of smaller peripheral lesions with improved instrument control, which leads to decreased variability in biopsy samples and improved accuracy.
The ACCESS study and the REACH Assessment used simulated tumors measuring 10 to 30 millimeters in cadaveric human lungs at tertiary or smaller segments that could be accessed with 97% accuracy while maintaining direct visualization.11,12
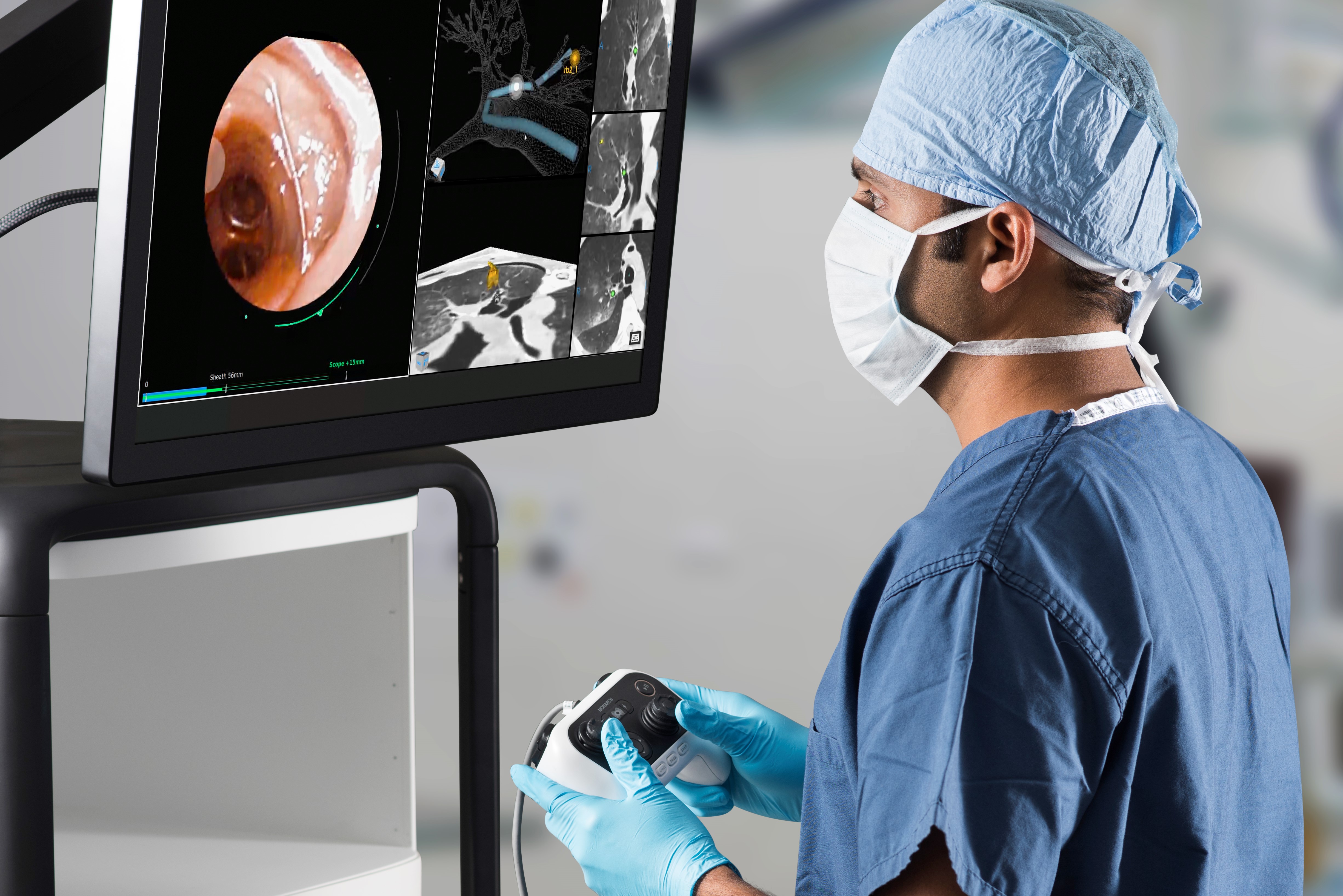
Auris Robotic Bronchoscope®
The Monarch™ robotic bronchoscope consists of a tower with monitor and articulating arms driven by navigational hand controller. (See Image 1A.) With electromagnetic guidance and a direct visualization telescoping outer scope sheath, the robotic controller allows for 360 degree movements and millimeter directional changes to accurately target a lesion. (See Images 1B, 1C and 1D.)
Constant direct vision allows for precise maneuvering to target a lesion. The inner sheath can then be locked into place allowing a reproducible approach to target lesion. This is especially important for those lesions that require traversing tortuous airways en route to the lesion. The handheld console has additional buttons to allow for airway suctioning and irrigation. This allows for continued visualization with live-time image capture to overcome technical obstacles such as blood or secretions in the airway that can interfere with lesion localization and biopsies.13,14
Ion Robotic Bronchoscope®
The Ion™ Endoluminal Platform uses a bronchoscope controlled by a single articulating arm. Much like the Monarch TM system, the bronchoscope of the Ion platform is controlled by a handheld controller.
Unique to the Ion is a joystick with a track ball controller. (See Image 2.) The joystick and track ball controller allows for precise movements in tortuous peripheral airways. With the use of a preplanned airway path, an ultrathin robotic catheter and a unique Flexision NeedleTM with 180 degrees of movement allows for multiple biopsies with slight changes in trajectory to optimize diagnostic yield.15 (See Image 3.)
Initial feasibility studies with the Monarch TM robotic platform by Rojas-Solano et al.16 investigated the rate of serious adverse events in a small patient sample size (15 patients) with a secondary endpoint of feasibility of robotic guided peripheral lung biopsy. No serious adverse events were noted in 93% of the patients. Additionally, time to biopsy improved from 45 minutes in the first five cases down to 20 minutes in the subsequent nine cases.
Larger retrospective multicenter follow-up studies17 had similar findings to the initial pilot study16 with the ability to reach 88.6% of peripheral lesions with a diagnostic yield of 69-77%. The pneumothorax rate remained low at 3.6% with airway bleeding found in only 2.4% of the cases.17,18
The PRECIsE Trial19 started in March of 2019 and is a prospective multicenter study to evaluate the clinical utility of the Ion Endoluminal System TM in peripheral lung nodule biopsies. The trial completion date is December 2021.
The primary endpoints of this open-label trial are time of procedure, biopsy success rate and sensitivity for diagnosis of malignancy on samples obtained through this system. Secondary outcomes include complication rates intra procedure, immediately post procedure and 10 days and 30-day post procedure.17
The future of robotic bronchoscopy and its role in diagnostic and therapeutic procedures is a robust and evolving field. The systems have already demonstrated increased rates of successful biopsies in tortuous airways without specific bronchus sign while maintaining direct visualization in these airways. Additionally, future applications include biopsies of multiple peripheral lesions.
The ability to navigate to previously inaccessible airways and stabilize catheters in them has laid the ground work for treatment modalities such as radiofrequency14,20 and cryoablative therapies. This novel evolving field in bronchoscopic technology provides new opportunities for rapid, highly accurate diagnostic and potential therapeutics in the landscape of lung cancer.
References:
- Torre, L.A., R.L. Siegel, and A. Jemal, Lung Cancer Statistics. Adv Exp Med Biol, 2016. 893: p. 1-19.
- Richardson, R.H., et al., The use of fiberoptic bronchoscopy and brush biopsy in the diagnosis of suspected pulmonary malignancy. Am Rev Respir Dis, 1974. 109(1): p. 63-6.
- Patz, E.F., et al., National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): design, intent, and availability of specimens for validation of lung cancer biomarkers. J Thorac Oncol, 2010. 5(10): p. 1502-6.
- Jin, F., et al., Severe complications of bronchoscopy. Respiration, 2008. 76(4): p. 429-33.
- Tajima, M., et al., CT Guided Needle Biopsy of Peripheral Lesions-Lesion Characteristics That May Increase the Diagnostic Yield and Reduce the Complication Rate. J Clin Med, 2021. 10(9).
- Gardner, D., et al., CT-guided transthoracic needle biopsy. Cardiovasc Intervent Radiol, 1991. 14(1): p. 17-23.
- Salazar, A.M. and J.L. Westcott, The role of transthoracic needle biopsy for the diagnosis and staging of lung cancer. Clin Chest Med, 1993. 14(1): p. 99-110.
- Shah, J., A. Vyas, and D. Vyas, The History of Robotics in Surgical Specialties. Am J Robot Surg, 2014. 1(1): p. 12-20.
- Folch, E.E., et al., Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol, 2019. 14(3): p. 445-458.
- Aboudara, M., et al., Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology, 2020. 25(2): p. 206-213.
- Chen, A.C., et al., Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respiration, 2020. 99(1): p. 56-61.
- Chen, A.C. and C.T. Gillespie, Robotic Endoscopic Airway Challenge: REACH Assessment. Ann Thorac Surg, 2018. 106(1): p. 293-297.
- Fielding, D.I.K., et al., First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration, 2019. 98(2): p. 142-150.
- Agrawal, A., D.K. Hogarth, and S. Murgu, Robotic bronchoscopy for pulmonary lesions: a review of existing technologies and clinical data. J Thorac Dis, 2020. 12(6): p. 3279-3286.
- Intuitive, I. www.intuitive.com/en-us/products-and service/ion/how-ion-works. 2021; Available from: www.intuitive.com/en-us/products-and service/ion/how-ion-works.
- Rojas-Solano, J.R., L. Ugalde-Gamboa, and M. Machuzak, Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J Bronchology Interv Pulmonol, 2018. 25(3): p. 168-175.
- Chaddha, U., et al., Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med, 2019. 19(1): p. 243.
- Kent, A.J., K.A. Byrnes, and S.H. Chang, State of the Art: Robotic Bronchoscopy. Semin Thorac Cardiovasc Surg, 2020. 32(4): p. 1030-1035.
- MD, H.L., PRECIsE: A Prospective Evaluation of the Clinical Utility for the Ion Endoluminal System. March 2019 to December 2021.
- Koizumi, T., et al., Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration, 2015. 90(1): p. 47-55.

